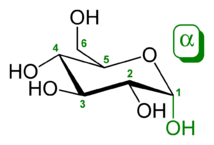Introduction to Carbohydrates
Carbohydrates are the most abundant class of organic compounds found in living organisms. They originate as products of photosynthesis, an endothermic reductive condensation of carbon dioxide requiring light energy and the pigment chlorophyll.
nCO2 + nH2O + Energy → CnH2nOn + nO2
As noted here, the formulas of many carbohydrates can be written as carbon hydrates, Cn(H2O)nCn(H2O)n, hence their name. The carbohydrates are a major source of metabolic energy, both for plants and for animals that depend on plants for food. Aside from the sugars and starches that meet this vital nutritional role, carbohydrates also serve as a structural material (cellulose), a component of the energy transport compound ATP/ADP, recognition sites on cell surfaces, and one of three essential components of DNA and RNA.
Carbohydrates are called saccharides or, if they are relatively small, sugars. Several classifications of carbohydrates have proven useful, and are outlined in the following table.
|
||||||
|
||||||
|
||||||
Original Source: http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Carbohydrates/Introduction_to_Carbohydrates
Saccharides
Monosaccharides are the simplest form of carbohydrates with only one simple sugar. They essentially contain an aldehyde or ketone group in their structure.[6] The presence of an aldehyde group in a monosaccharide is indicated by the prefix aldo-. Similarly, a ketone group is denoted by the prefix keto-.[1] Examples of monosaccharides are the hexoses glucose, fructose, and galactose and pentoses, ribose, and deoxyribose Consumed fructose and glucose have different rates of gastric emptying, are differentially absorbed and have different metabolic fates, providing multiple opportunities for 2 different saccharides to differentially affect food intake.[6] Most saccharides eventually provide fuel for cellular respiration.
Disaccharides are formed when two monosaccharides, or two single simple sugars, form a bond with removal of water. They can be hydrolyzed to yield their saccharin building blocks by boiling with dilute acid or reacting them with appropriate enzymes.[1] Examples of disaccharides include sucrose, maltose, and lactose.
Polysaccharides are polymerized monosaccharides, or complex carbohydrates. They have multiple simple sugars. Examples are starch,cellulose, and glycogen. They are generally large and often have a complex branched connectivity. Because of their size, polysaccharides are not water-soluble, but their many hydroxy groups become hydrated individually when exposed to water, and some polysaccharides form thick colloidal dispersions when heated in water.[1] Shorter polysaccharides, with 3 - 10 monomers, are called oligosaccharides.[7] A fluorescent indicator-displacement molecular imprinting sensor was developed for discriminating saccharides. It successfully discriminated three brands of orange juice beverage.[8] The change in fluorescence intensity of the sensing films resulting is directly related to the saccharide concentration.[9]
Original Source: https://en.wikipedia.org/wiki/Biomolecule
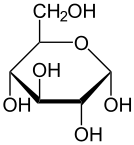
Glucose
Glucose is a sugar with the molecular formula C6H12O6. The name "glucose" comes from the Greek word γλυκός, meaning "sweet wine, must".[3] The suffix "-ose" is a chemical classifier, denoting a carbohydrate. It is also known as grape sugar. With 6 carbonatoms, it is classed as a hexose, a sub-category of monosaccharides. α-D-glucose is one of the 16 aldose stereoisomers. The D-isomer (D-glucose), also known as dextrose, occurs widely in nature, but the L-isomer (L-glucose) does not. Glucose is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is a very important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen, for times when the organism will need it. Glucose circulates in the blood of animals as blood sugar. Glucose can be obtained by hydrolysis of carbohydrates such as milk, cane sugar, maltose, cellulose, glycogen etc. It is however, manufactured by hydrolysis of cornstarch by steaming and diluting acid.[4]
Original Source: https://en.wikipedia.org/wiki/Glucose
Introduction to Proteins
Proteins are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within livingorganisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.
Biochemistry
Most proteins consist of linear polymers built from series of up to 20 different L-α-amino acids. All proteinogenic amino acids possess common structural features, including an α-carbon to which an amino group, a carboxyl group, and a variable side chain are bonded. Only proline differs from this basic structure as it contains an unusual ring to the N-end amine group, which forces the CO–NH amide moiety into a fixed conformation.[1] The side chains of the standard amino acids, detailed in the list of standard amino acids, have a great variety of chemical structures and properties; it is the combined effect of all of the amino acid side chains in a protein that ultimately determines its three-dimensional structure and its chemical reactivity.[2] The amino acids in a polypeptide chain are linked by peptide bonds. Once linked in the protein chain, an individual amino acid is called aresidue, and the linked series of carbon, nitrogen, and oxygen atoms are known as themain chain or protein backbone.[3]
The peptide bond has two resonance forms that contribute some double-bond character and inhibit rotation around its axis, so that the alpha carbons are roughly coplanar. The other two dihedral angles in the peptide bond determine the local shape assumed by the protein backbone.[4] The end of the protein with a free carboxyl group is known as the C-terminus or carboxy terminus, whereas the end with a free amino group is known as the N-terminus or amino terminus. The words protein, polypeptide, and peptide are a little ambiguous and can overlap in meaning. Protein is generally used to refer to the complete biological molecule in a stable conformation, whereas peptide is generally reserved for a short amino acid oligomers often lacking a stable three-dimensional structure. However, the boundary between the two is not well defined and usually lies near 20–30 residues.[5] Polypeptide can refer to any single linear chain of amino acids, usually regardless of length, but often implies an absence of a defined conformation.
Protein Structure
Most proteins fold into unique 3-dimensional structures. The shape into which a protein naturally folds is known as its native conformation.[12] Although many proteins can fold unassisted, simply through the chemical properties of their amino acids, others require the aid of molecular chaperones to fold into their native states.[13] Biochemists often refer to four distinct aspects of a protein's structure:[14]
- Primary structure: the amino acid sequence. A protein is a polyamide.
- Secondary structure: regularly repeating local structures stabilized by hydrogen bonds. The most common examples are the α-helix, β-sheet and turns. Because secondary structures are local, many regions of different secondary structure can be present in the same protein molecule.
- Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structures to one another. Tertiary structure is generally stabilized by nonlocal interactions, most commonly the formation of a hydrophobic core, but also through salt bridges, hydrogen bonds, disulfide bonds, and evenposttranslational modifications. The term "tertiary structure" is often used as synonymous with the term fold. The tertiary structure is what controls the basic function of the protein.
- Quaternary structure: the structure formed by several protein molecules (polypeptide chains), usually called protein subunits in this context, which function as a singleprotein complex.
Proteins are not entirely rigid molecules. In addition to these levels of structure, proteins may shift between several related structures while they perform their functions. In the context of these functional rearrangements, these tertiary or quaternary structures are usually referred to as "conformations", and transitions between them are called conformational changes. Such changes are often induced by the binding of a substrate molecule to an enzyme'sactive site, or the physical region of the protein that participates in chemical catalysis. In solution proteins also undergo variation in structure through thermal vibration and the collision with other molecules.[15]
Proteins can be informally divided into three main classes, which correlate with typical tertiary structures: globular proteins, fibrous proteins, and membrane proteins. Almost all globular proteins are soluble and many are enzymes. Fibrous proteins are often structural, such as collagen, the major component of connective tissue, or keratin, the protein component of hair and nails. Membrane proteins often serve as receptors or provide channels for polar or charged molecules to pass through the cell membrane.[16]
Original Source: https://en.wikipedia.org/wiki/Protein
Introduction to Lipids
Lipids (oleaginous) are chiefly fatty acid esters, and are the basic building blocks of biological membranes. Another biological role is energy storage (e.g., triglycerides). Most lipids consist of a polar or hydrophilic head (typically glycerol) and one to three nonpolar or hydrophobic fatty acid tails, and therefore they are amphiphilic. Fatty acids consist of unbranched chains of carbon atoms that are connected by single bonds alone (saturated fatty acids) or by both single and double bonds (unsaturated fatty acids). The chains are usually 14-24 carbon groups long, but it is always an even number.
For lipids present in biological membranes, the hydrophilic head is from one of three classes:
- Glycolipids, whose heads contain an oligosaccharide with 1-15 saccharide residues.
- Phospholipids, whose heads contain a positively charged group that is linked to the tail by a negatively charged phosphate group.
- Sterols, whose heads contain a planar steroid ring, for example, cholesterol.
Other lipids include prostaglandins and leukotrienes which are both 20-carbon fatty acyl units synthesized from arachidonic acid. They are also known as fatty acids
Original Source: https://en.wikipedia.org/wiki/Biomolecule