Fermentation is a metabolic process that converts sugar to acids, gases, or alcohol. It occurs in yeast and bacteria, and also in oxygen-starved muscle cells, as in the case of lactic acid fermentation. Fermentation is also used more broadly to refer to the bulk growth of microorganisms on a growth medium, often with the goal of producing a specific chemical product. French microbiologist Louis Pasteur is often remembered for his insights into fermentation and its microbial causes. The science of fermentation is known as zymology.
Fermentation takes place when the electron transport chain is unusable (often due to lack of a final electron receptor, such as oxygen), and becomes the cell’s primary means of ATP (energy) production.[1] It turns NADH and pyruvateproduced in glycolysis into NAD+ and an organic molecule (which varies depending on the type of fermentation; see examples below). In the presence of O2, NADH and pyruvate are used to generate ATP in respiration. This is called oxidative phosphorylation, and it generates much more ATP than glycolysis alone. For that reason, cells generally benefit from avoiding fermentation when oxygen is available, the exception being obligate anaerobes which cannot tolerate oxygen.
The first step, glycolysis, is common to all fermentation pathways this is the cause of fermentation:
- C6H12O6 + 2 NAD+ + 2 ADP + 2 Pi → 2 CH3COCOO− + 2 NADH + 2 ATP + 2 H2O + 2H+
Pyruvate is CH3COCOO−. Pi is inorganic phosphate. Two ADP molecules and two Pi are converted to two ATP and two water molecules via substrate-level phosphorylation. Two molecules of NAD+ are also reduced to NADH.[2]
In oxidative phosphorylation the energy for ATP formation is derived from an electrochemical proton gradientgenerated across the inner mitochondrial membrane (or, in the case of bacteria, the plasma membrane) via the electron transport chain. Glycolysis has substrate-level phosphorylation (ATP generated directly at the point of reaction).
Humans have used fermentation to produce food and beverages since the Neolithic age. For example, fermentation is used for preservation in a process that produces lactic acid as found in such sour foods as pickled cucumbers, kimchiand yogurt (see fermentation in food processing), as well as for producing alcoholic beverages such as wine (seefermentation in winemaking) and beer. Fermentation can even occur within the stomachs of animals, such as humans.
Original Source: https://en.wikipedia.org/wiki/Fermentation
Ethanol Fermentation
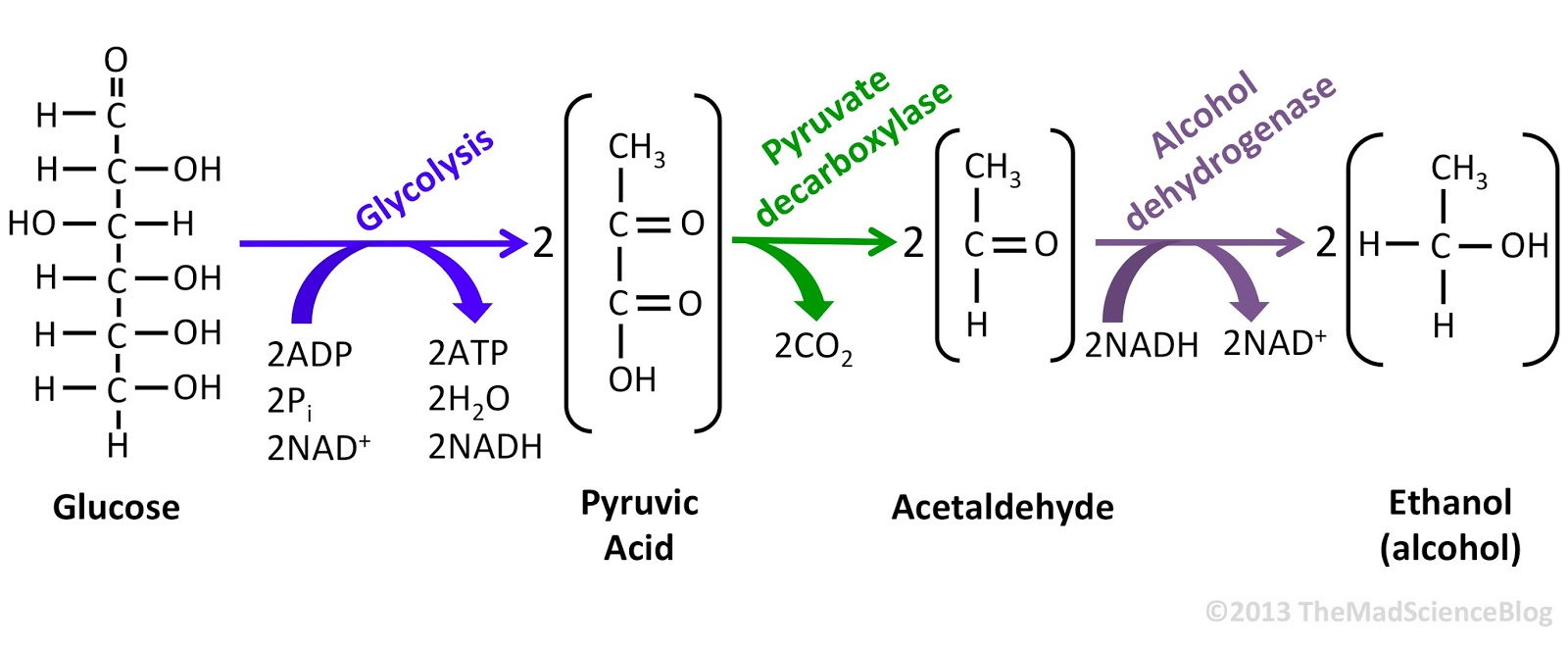
Ethanol fermentation, also called alcoholic fermentation, is a biological process which convertssugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as a side-effect. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process.
Ethanol fermentation has many uses, including the production of alcoholic beverages, the production of ethanol fuel, and bread baking.
Biochemical process of fermentation of sucrose
The chemical equations below summarize the fermentation of sucrose (C12H22O11) into ethanol (C2H5OH). Alcoholic fermentation converts one mole of glucose into two moles of ethanol and two moles of carbon dioxide, producing two moles ofATP in the process.
The overall chemical formula for alcoholic fermentation is:
- C6H12O6 → 2 C2H5OH + 2 CO2
Sucrose is a dimer of glucose and fructose molecules. In the first step of alcoholic fermentation, the enzyme invertase cleaves the glycosidic linkage between the glucose and fructose molecules.
- C12H22O11 + H2O + invertase → 2 C6H12O6
Next, each glucose molecule is broken down into two pyruvate molecules in a process known as glycolysis.[1] Glycolysis is summarized by the equation:
- C6H12O6 + 2 ADP + 2 Pi + 2 NAD+ → 2 CH3COCOO− + 2 ATP + 2 NADH + 2 H2O + 2 H+
The chemical formula of pyruvate is CH3COCOO−. Pi stands for the inorganic phosphate.
The enzyme in step 3 is pyruvate decarboxylase.
As shown by the reaction equation, glycolysis causes the reduction of two molecules of NAD+ to NADH. Two ADP molecules are also converted to two ATP and two water molecules via substrate-level phosphorylation.
Original Source: https://en.wikipedia.org/wiki/Ethanol_fermentation
 Original Source: http://www.themadscienceblog.com/2013/05/biology-and-beer.html
Original Source: http://www.themadscienceblog.com/2013/05/biology-and-beer.html

